Artificial photosynthetic solar fuels and foodstuffs are an effective and attractive approach for sustaining our society in a green and low-carbon manner. Although it is a big challenge to develop science and technology of solar energy conversion, solar fuels including green hydrogen and liquid sunshine such as methanol produced via artificial photosynthesis are an important pathway to reduce the dependence on fossil fuels and the emission of carbon dioxide [1]. Artificial photosynthetic systems aim to the efficient conversion of solar energy with water and carbon dioxide into the stable, energy-dense carriers for chemical industrial supply chains. Furthermore, the advanced foodstuffs, such as biological macromolecules including starch and protein via artificial photosynthesis, will play an important role in animal feed and food industrial feedstock in the future. Therefore, artificial photosynthetic technologies for carbon dioxide conversion and utilization have shed light on the roadmap to move forward to carbon neutrality.
In natural photosynthesis, photosystems (photosystem II and photosystem I) on the thylakoid membrane harvest sunlight to drive the water oxidation for dioxygen formation and produce the reducing equivalents (NADPH and ATP). Subsequently, the reducing power is used for the fixation of carbon dioxide through Calvin cycle to synthesize the primary molecule, glyceraldehyde 3-phosphate (GAP), which is used for the subsequent process of biomass production. When the GAP is accumulated in the chloroplast, it is prone to form starch granules, which is utilized for energy storage. GAP is also the substrate for protein synthesis. Namely, nature offers the pathways for the synthesis of starch and protein from solar energy. However, the efficiency of energy conversion and reaction yield of the aimed product is insufficient to satisfy the requirements of our increasing demand.
Nature affords scientists lessons that guide us to pursue the fundamental way of exploring artificial photosynthetic approach for the synthesis of glucose from water, carbon dioxide, and sunlight. Artificial photosynthetic systems can conduct efficient conversion of carbon dioxide into energy carriers utilizing sunlight, while biological enzymes are expert in the construction of anabolic pathway to build blocks from simple molecules into macromolecules (starch, proteins, etc.). Therefore, combination of artificial photosynthetic with biological enzymatic catalysis can overcome the intrinsic limitation of the single component to achieve a unique function with an outstanding performance. However, the coupling of artificial and biological processes faces much difficulty for efficient integration due to the crossover interaction and the mismatch of thermodynamics or kinetics. Consequently, the hybrid systems coupling chemical and biological components is a highly attractive and promising for the synthesis of biobased fuels and foodstuffs.
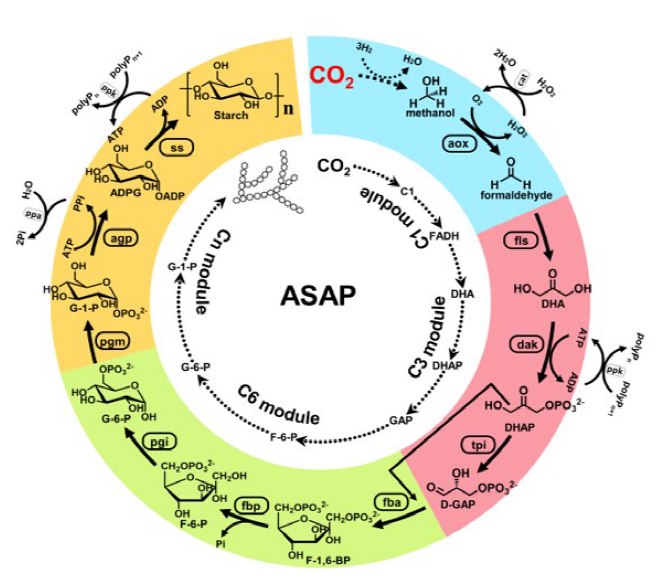
Inspired by nature, according to the framework that couples artificial light reaction and biological dark reaction, Ma’s group cooperated with Li’s group recently developed a pathway combining artificial photosynthetic with multi-cascade enzymatic processes for starch synthesis from carbon dioxide [2]. Based on the calculation and analysis of enzyme element database, the pathways of starch synthesis can be theoretically designed on the account of energy requirement and reaction kinetics. As a result, it was confirmed experimentally that using the energy carrier methanol as the initial building block, the optimized pathway for the synthesis of starch achieved with the shortest steps (Fig. 1). Methanol molecule as the energy carrier and carbon skeleton was produced by artificial photosynthetic system from CO2. Therefore, artificial starch anabolic pathway (ASAP) contained two processes of (I) solar energy conversion into methanol from CO2 by artificial photosynthesis and (II) architecture of starch molecule by biological enzyme catalysis from methanol. ASAP, driven by solar hydrogen, converted CO2 to starch at a rate of 22 nanomoles of CO2 per minute per milligram of total catalyst, an 8.5-fold higher rate than starch synthesis in maize.
Fig. 1.
Fig. 1.
Scheme of artificial starch anabolic pathway. Inner circle: schematic of the artificial starch pathway drafted by computational pathway design with divided modules. C1 here indicates formic acid and methanol. Outer circle: schematic of artificial starch anabolic pathway (ASAP) 1.0, with individual modules colored. Auxiliary enzymes and chemicals are indicated. ADPG, ADP glucose; aox, alcohol oxidase; FADH, formaldehyde; F-1,6-BP, D-fructose-1,6-bisphosphate; F-6-P, D-fructose-6- phosphate; GAP, D-glyceraldehyde 3-phosphate; pgi, phosphoglucose isomerase; polyP, polyphosphate; pgm, phosphoglucomutase; ppa, pyrophosphatase; ppk, polyphosphate kinase; ss, starch synthase; tpi, triosephosphate isomerase. Reprinted with permission from Ref. [2]. Copyright 2021, Science.
The (I) process of liquid sunshine is conducted by artificial photosynthesis group led by Prof. Can Li from Dalian Institute of Chemical Physics, CAS. The process package is composed of three crucial steps. First, solar light is captured and converted into electricity by photovoltaics; Second, electrolysis of water is carried out by a highly efficient and stable electrocatalyst for oxygen and green hydrogen production; Finally, methanol is synthesized via carbon dioxide hydrogenation by a highly selective and stable ZnO-ZrO2 solid solution catalyst [3]. The catalyst can achieve methanol selectivity of up to 86 to 91% with CO2 single-pass conversion of more than 10% under reaction conditions of 5.0 MPa, 24000 mL/(g h), H2/CO2 = 3/1 to 4/1, 315 to 320 °C. Overall, the sunlight is converted into chemical energy and stored into methanol through water splitting and carbon dioxide hydrogenation reactions. Furthermore, the technology has been put into practice at large scale with a production capability of thousand tons. Liquid sunshine methanol that serves as energy and carbon source is in the center to link (I) with (II) processes for starch synthesis.
The (II) process of starch pathway construction is conducted by Ma’s group from Tianjin Institute of Industrial Biotechnology, CAS. The designed pathway is a multi-cascade enzymatic reaction process including a C1 module (for formaldehyde production), a C3 module (for D-glyceraldehyde 3-phosphate production), a C6 module (for D-glucose-6-phospate production), and a Cn module (for starch synthesis). As methanol has a higher Gibbs free energy, the following steps from C1 to Cn are favorable thermodynamically. The adenosine 5′-triphosphate (ATP) supply is used to activate the substrates. Methanol serves as both energy supplier and building block. The crucial step is the following: methanol produced from CO2 is oxidized to formaldehyde by alcohol oxidase (aox); condensation of formaldehyde forms the dihydroxyacetone (DHA) by formolase (fls); DHA is converted into the glyceraldehyde 3-phosphate (GAP), which is the key building block for the main metabolism in the biological system; GAP and DHA substrates are coupled for the glucose-6-phospate production; finally, amylose and amylopectin polymers are synthesized by forming α-1,4-glycosidic bonds or α-1,6-glycosidic bonds.
Although the rational designed pathway for starch synthesis is scientifically important and is considered as new technology impact on the world, there are still much room focused on the system to improve the efficiency and stability for the application of the pathway. Because the different enzymatic kinetics led to the mismatch between the modules, the cascade reactions will be limited by one of the substrates. For example, condensation of formaldehyde by the fls enzyme requires a high concentration of the formaldehyde, but the oxidation rate of methanol to formaldehyde is quite slow. This gave rise to the difficulty to start the condensation reaction up. On the other hand, regulation of metabolic flow to keep different reactions in balance is indispensable to the competition of catalytic reactions. Besides, the inhibition from side products should be liberated by improving the performance of enzymes. Therefore, more efficient and stable enzymes with total system integration solutions are needed to be developed to overcome these obstacles for the starch synthesis.
In conclusion, a biohybrid artificial photosynthetic system is developed for the starch synthesis from carbon dioxide. The unit of liquid sunshine is responsible for methanol production from CO2 reduction by green hydrogen in the source of water electrolysis utilizing solar energy. Subsequently, in the unit of biological synthesis, methanol is converted by engineered recombinant enzymes first to three and six carbon sugar modules and then to polymeric starch. The present approach opens the way that allows it to harness the strengths inherent to both artificial photosynthesis and synthetic biology for the green and low-carbon biomanufacturing of starch.
Acknowledgements
This work was conducted by the Fundamental Research Center of Artificial Photosynthesis (FReCAP), financially supported by National Natural Science Foundation of China(22088102).
Reference